Disclaimer: I do NOT think that women should be blamed or targeted for taking antidepressants during pregnancy. Antidepressants are absolutely necessary for many people to be mentally and physically healthy individuals. I am pointing out that there may be unappreciated health consequences to antidepressant use during pregnancy, and that these should be part of the conversation.
As I discussed last time, antidepressant use has skyrocketed in the past 25 years, and about 1 in 8 pregnant women in America take antidepressants. However, the developmental effects that this may have on children (and future generations) are unknown, though the existing research is troubling. While there are many possible ways to think about this problem, an interesting lens through which we can view it is epigenetics.
Your DNA contains all of the information needed to be human. However, that information isn’t used all at one time. For example, you don’t need to produce liver enzymes in the skin. Different pieces (genes) are deployed when the body deems it appropriate through a complex regulatory system (click here for a nice video explanation of this system). Epigenetic modifications modulate gene expression up, down, on, or off, similar to the way software uses different functions on a computer.
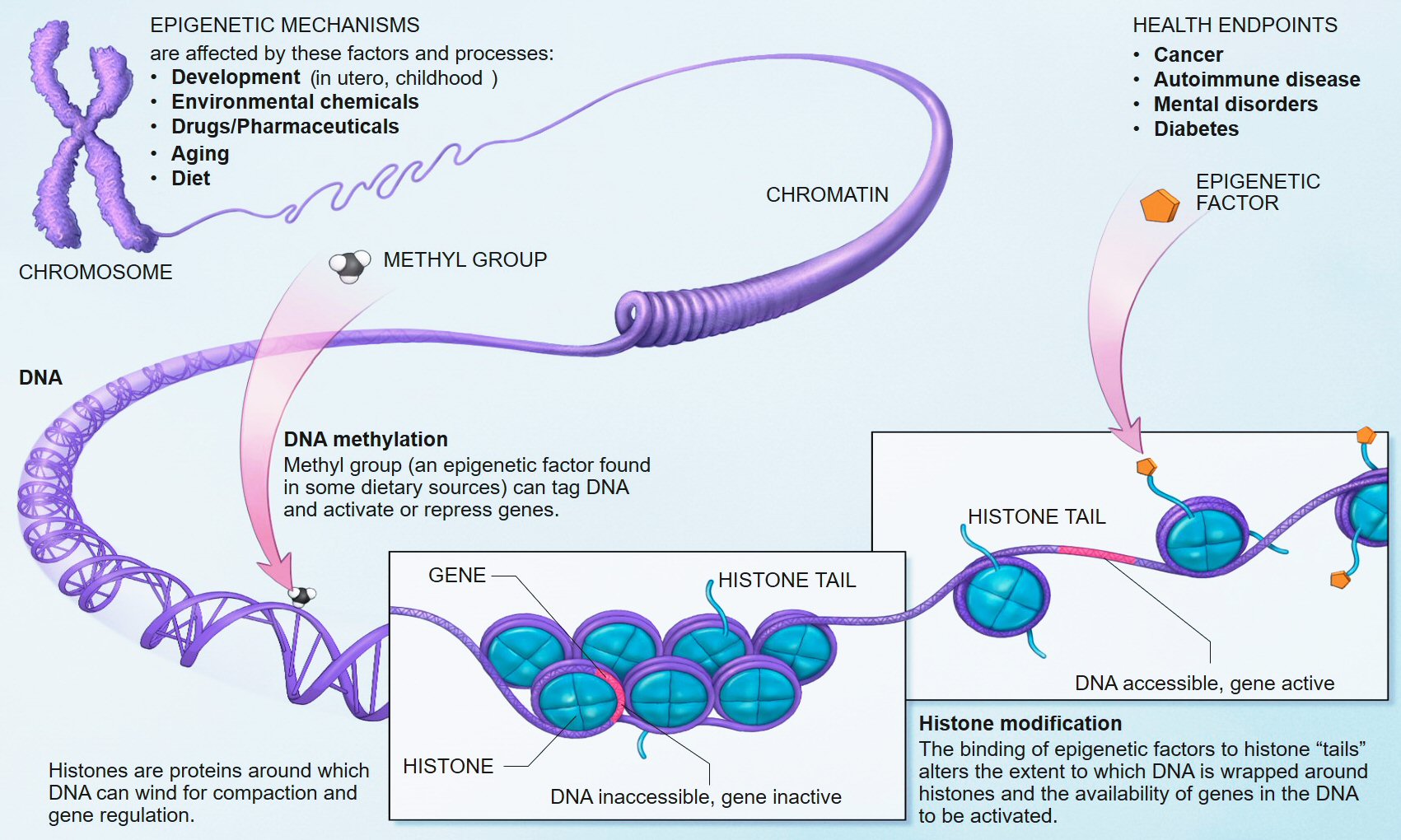
Some of the epigenetic mechanisms at play. Source: Wikipedia
Although not all these modifications are permanent, some can last for a lifetime and even be passed down to future generations.
When the sperm and the egg meet, many of the epigenetic modifications from each parent are erased, and new epigenetic modifications occur as the fetus develops. This is necessary, since the cells in the developing fetus need maximum flexibility to differentiate into the marvelously complex parts of the human body and to adapt to the prenatal environment. Despite being a time of great potential, this time of fetal development is fraught with danger.

Source: Time Magazine
In recent years, a new paradigm for thinking about prenatal exposures called Developmental Origins of Health and Disease (or DOHaD) has become an increasingly visible field of study. DOHaD is the concept that prenatal exposure to heavy metals, malnutrition, antibiotics, or other hazards can cause epigenetic, microbiome, and other physiological changes linked to higher risks of disease later in life.
For example, when a fetus is malnourished, it may develop a “thrifty” phenotype. In other words, the body adapts to survive in environments scarce in food and nutrients. After birth, if the child grows up with abundant food, the mismatch between their prenatal and postnatal environments can put them at higher risk of diseases such as diabetes and obesity.
So what happens later in life when a child develops in a prenatal environment with antidepressants? Furthermore, what effects might this have in the longer term – not just the life of the exposed child, but in their children, and their children’s children? These questions are being explored with in vitro fertilization and autism, and could be expanded to antidepressants as well.
Could the rapidly increasing prevalence of depression be tied to widespread use of antidepressants? The time scale of the increase does not fit with slow evolutionary changes like mutation and genetic drift, suggesting the possibility of an epigenetic mechanism. (There are many other factors at play besides antidepressants, however; read this great review article for other key factors.)
Babies who were exposed to antidepressants during the third trimester often experience symptoms of withdrawal. Antidepressants affect the uptake of specific neurotransmitters, like serotonin, and likely have epigenetic effects. We know that parenting style (in rats) affects stress and serotonin regulation: more grooming and licking is linked to less fearful and less stressed out pups, and the changes are reversible. Is it possible to reverse prenatal effects with targeted behavioral therapies?
If the prenatal environment has overabundant serotonin, does this modify the brain chemistry of a child to expect unusually high amounts of serotonin? What effects might that have?
What if serotonin is the wrong target altogether? Is there any risk associated with fathers on antidepressants during conception?
Women often need to increase their doses of antidepressants in weeks 24-30 of pregnancy to maintain treatment efficacy, when the fetal brain is growing rapidly. Is this something to be worried about, or is it benign?
We don’t know the answers to these questions; we need more data. However, the data that we do have (along with the plausible potential mechanisms) suggests that these questions could use a deeper look.
Tune in next time for a lively discussion of possible ways to address these questions!
References
Perera, Frederica, and Julie Herbstman. “Prenatal environmental exposures, epigenetics, and disease.” Reproductive toxicology 31.3 (2011): 363-373. DOI
Gillman, Matthew W. “Developmental origins of health and disease.” The New England journal of medicine 353.17 (2005): 1848. link
Tang, Wan-yee, and Shuk-mei Ho. “Epigenetic reprogramming and imprinting in origins of disease.” Reviews in Endocrine and Metabolic Disorders 8.2 (2007): 173-182. DOI
Hales, C. Nicholas, and David JP Barker. “The thrifty phenotype hypothesis.”British medical bulletin 60.1 (2001): 5-20. link
Vialou, Vincent, et al. “Epigenetic Mechanisms of Depression and Antidepressants Action.” Annual review of pharmacology and toxicology 53 (2013): 59. DOI
Hudak, Mark L., et al. “Neonatal drug withdrawal.” Pediatrics 129.2 (2012): e540-e560. DOI
Meaney, Michael J., and Moshe Szyf. “Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome.” Dialogues in clinical neuroscience 7.2 (2005): 103. link
Mattison, Donald, and Anne Zajicek. “Gaps in knowledge in treating pregnant women.” Gender medicine 3.3 (2006): 169-182. DOI
